Data provider
Budapest University of Technology and Economics, Department of Applied Biotechnology and Food Science, Environmental Microbiology and Biotechnology Group
Contact details
Compulsory sheet
Information on the method
Measured endpoint
Description of the measurement technique
Measuring range
- Metals, semi-metals and their compounds
Measuring range
- Petroleum derivatives (TPH)
Measuring range
- Halogenated aliphatic organic compounds
Measuring range
- Other organic chemical substance
Implementation conditions
Implementation costs
Innovation, main features
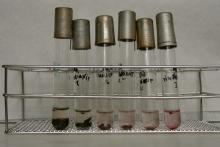
Whole soil instead of soil extract ensures direct contact with the test organism.Respiration inhibition due to toxic compounds is measured by dehydrogenase enzyme activity. The dehydrogenase activity is indicated by colour change. An alternative electron acceptor, TTC is added to the tested soil suspension. The colour change is due to the formation of formasane from TTC. The resulted red colour can be detected either visually in the soil suspension or measured by photometer after solvent extraction.
In case of unknown contaminants and contaminant mixtures are in the soil. If the contaminant is not bioavailable and it is expected that the bioavailability changes in function of time and conditions. In case it is important that during the test the test microorganisms are in contact with the tested soil or it is important that any other decisive interactions take place.
The presence of soil suspension may hinder the quality of visual evaluation.
Azomonas agilis is kept on Fjodorov agar medium. After innoculation it is incubated at 28 ºC for 48 hours. The test requires that Azomonas agilis is innoculated into 30 ml sterile Fjodorov substrate. The innoculum is shaked for 72 hours at 28 ºC. 2-2 grams of the soil samples are sterilised in steam. A five series two-fold dilution series (0.5 g, 0.25 g, 0.125 g, 0.0625 g and 0.0312 g) is prepared in test tubes in sterile conditions. Other microorganisms may inhibit Azomonas agilis reproduction. 100 cm3 of sterile Fjodorov substrate and 1 cm3 of sterile TTC solution is added to 5 cm3 bacterium suspension shaken for 72 hours at 28 ºC. 2-2 cm3 of the prepared mixture is pipetted into the soil containing test tubes, then it is homogenised (by Vortex) and incubated for 72 hours at 28 ºC. As reference 400, 40, 4, 0.4 and 0.04 ppm Cu dilution series is used. Evaluation is done visually after 72 hours. Occurrence of red colour indicates microbial activity. In the presence of toxic metals dehydrogenase enzyme activity is inhibited or it is lower than the dehydrogenase activity of the uncontaminated control sample, therefore TTC is not reduced and consequently there is no red colour visible. When red colour is not detected the inhibition of the enzyme activity is 100%, when the red colour is only slightly visible the enzyme activity inhibition is 50%. The red colour indicates microbial activity and no inhibition. The inhibition values are plotted function of the soil dose. The soil dose relevant to 50% enzyme activity inhibition is read on the resulted dose-response curve showing the soil amount (in grams) that reduced the enzyme activity to the half.
SWOT (evalaution based on scores)
SWOT (evaluation in words)
The used test organism and direct contact test are sensitive not only to the toxic metals but also to PAH and PCBs. Given that the test organism is a soil living organism one gets a realistic picture about the effect of contaminants on the soil ecosystem.
The sensitivity of the test organism has to be controlled. The endpoint, the detected red colour may be subjective and difficult to evaluate in case of brown coloured soils that do not settle in water suspension.
The test results can be evaluated not only visually. The metabolism product resulting the red colour can be extracted by solvent and subjected to photometry. This way the results are more objective and quantitative.
The sample has to be sterilised. The soil sterilisation method has to be carefully selected taking into account the physical-chemical effects ( evaporation, decomposition, new toxic decomposition products) of the sterilisation method.
Other information, references
In case of high Kow contaminants and synergic effects the results of the tests on soil suspension show higher toxicity as compared to the water extracts of the same soil. For this reason this test is more conservative (within a realistic limit) than testing the water extract. Some contaminant-soil interactions may be so strong that due to the matrix effect of the soil the soil suspension test shows slighter effect than in case of water extract or leachate.
Gruiz, Horváth és Molnár (2001)Környezettoxikológia – Vegyi anyagok hatása az ökoszisztémára, Műegy.Kiadó, Bp.
Gruiz, K. (2005) Soil testing triad for contaminated soil – In: Soil Remediation No6. (Eds.Fava and Canepa) pp.45–70, INCA, It
Feigl, V., Atkári, Á., Anton, A., Gruiz, K. (2007) Chemical stabilisation combined with phytostabilisation, Adv. Mat. Res. 20–21, 315–318.
Feigl V., Atkári Á., Uzinger N., Gruiz K. (2006) Fémmel szennyezett területek integrált kémiai és fitostabilizációja, Orsz. Körny.véd. Konf. Kiadványa 99–108.
Completed applications
The test may be used to monitor the phytoremediation technology, namely the toxic metal stabilisation effect of the applied additive.