Data provider
Budapest University of Technology and Economics, Department of Applied Biotechnology and Food Science, Environmental Microbiology and Biotechnology Group
Contact details
Compulsory sheet
Information on the method
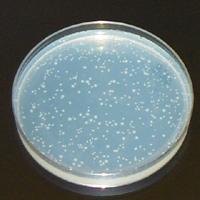
Measured endpoint
Description of the measurement technique
Measuring range
Implementation conditions
Implementation costs
Innovation, main features
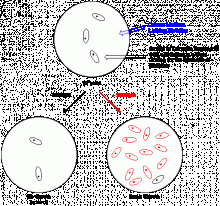
The histidine operon of the Salmonella bacterial test strain used in this test has various mutations, for which reason these strains cannot syntetise hystidine, therefore their growth is inhibited unless the damaged gene sequence of the test organism is restored by a new mutation or reverse mutation.
The Ames test is a quick, reverse-mutation test able to screen the mutagenic effects of chemicals. It is used to replace or precede animal tests. If the Ames test is positive than the chemical is mutagenic. If the Ames test is negative the chemical may be mutagenic or carcingenic to humans and animals.
Interpretation of the results is difficult without having the possibility to compare them with animal tests. Nowdays there are many results available so that the Salmonella can be used as a model system. Even this way one is faced with contradictions ex. Pentachlorphenol proved to be mutagenic and carcinogenic in case of animal tests and negative in Ames test.
ISO 16240:2005
SWOT (evalaution based on scores)
SWOT (evaluation in words)
It does not need special equipment. It can replace expensive, laborious and ethically questionable animal tests. The statistics of the measurement can be improved because it allows many repetitions.
The mutation is a statistical phenomenon, it is difficult to be reproduced accurately and it needs more parallel measurements. However, based on the concentration dependance of the mutations the positive effect is obvious allowing also statistical evaluation.
Mutagenicity and carcinogenicity can be assessed also based on the Ames test results without animal test. Activation of certain chemicals during human digestion can be modelled by in vitro pre-treatment. The mixture of test-organisms containing various mutations is able to detect the effect of mutagenic chemicals of various mechanism.
The conformity of mammal and Ames test results is approximately 80% meaning that in some cases mutagenicity is underestimated while in other cases overestimated.
Other information, references
Some chemicals do not appear to be mutagenic although the animal tests proved to be mutagenic and carcinogenic. In case the contaminant and its mutation effect mechanisms are unknown, the other Salmonella mixture is to be used in order to avoid the false negative result.
Maron, D. M.; Ames, B. N.: Revised methods for the Salmonella mutagenicity test Mutation Research, 113, pp. 173-215, 1983
Completed applications
The water solution of pentachlorphenol (PCP) did not indicate mutagenicity.
The pretreatment modelling digestion did not result mutagenicity in case of PCP.
The test results of the cyclodextrine-PCP complex were positive. See also datasheet No. 314 on the use of cyclodextrine for soil mutagenicity test.